Superior decision making
Treatment selection guidance across therapy classes
Strata Select combines simultaneous DNA and quantitative RNA sequencing from a single small tumor tissue sample to provide treatment selection guidance for immunotherapy as well as other classes of therapy.
Immunotherapy Response Score
- Pan-solid tumor diagnostic tool
- Predicts anti-PD-1/PD-L1 monotherapy benefit
Genomic Signatures
- Tumor mutation burden (TMB)
- Microsatellite instability (MSI)
Comprehensive Genomic Profiling
- DNA and RNA sequencing analysis across 437 genes
- Guidance for genomic-alteration targeted therapies
Supplemental Insights
- Quantitative RNA sequencing-based guidance for expression-based therapies like antibody-drug conjugates
- Homologous recombination deficiency (HRD) results for ovarian cancer patients
PD-L1 IHC

Clear reports with the context you need
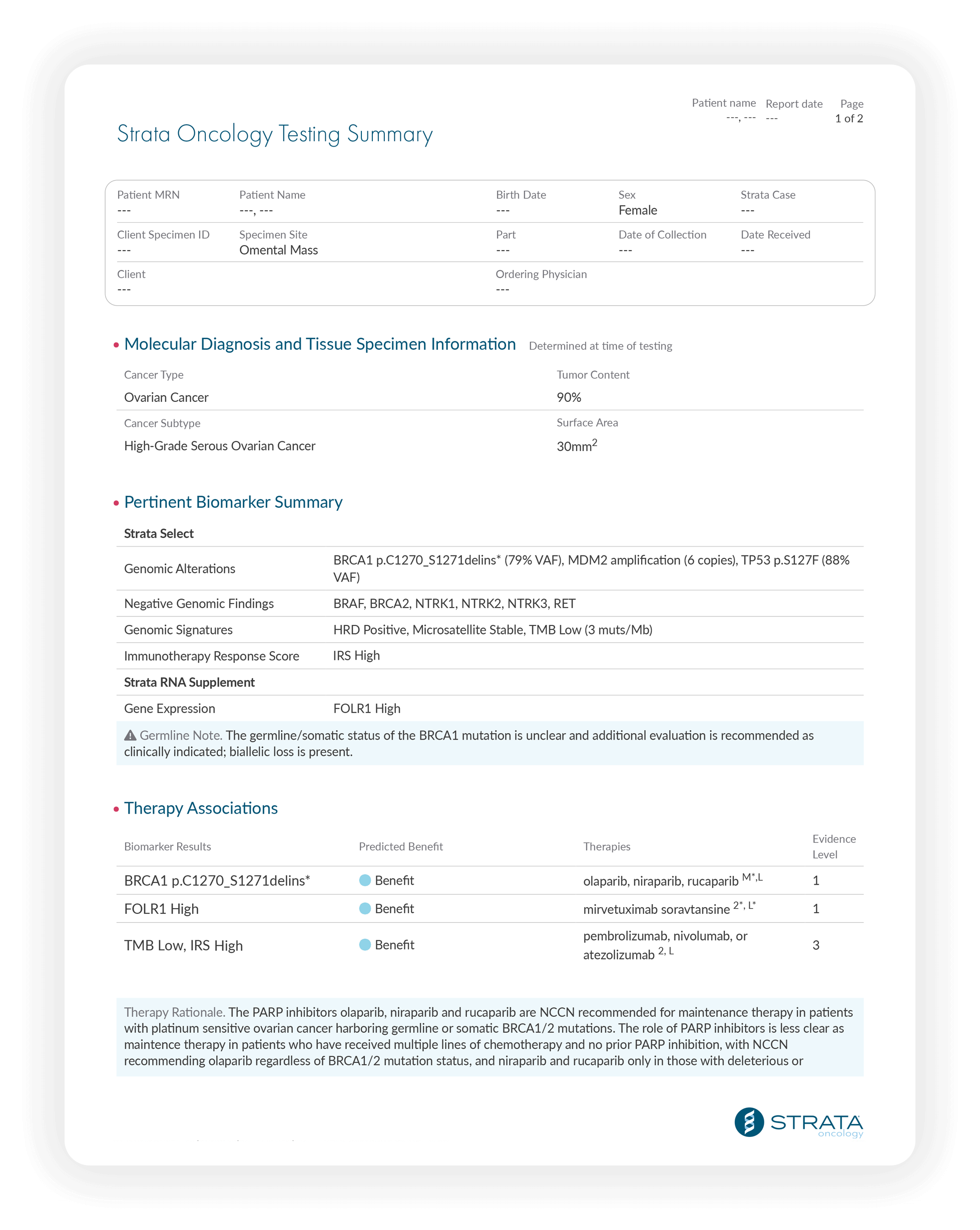
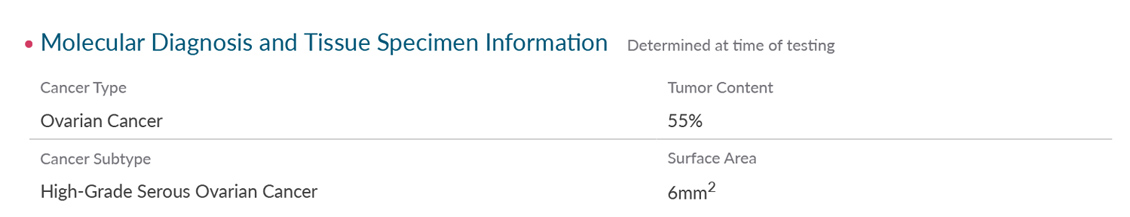
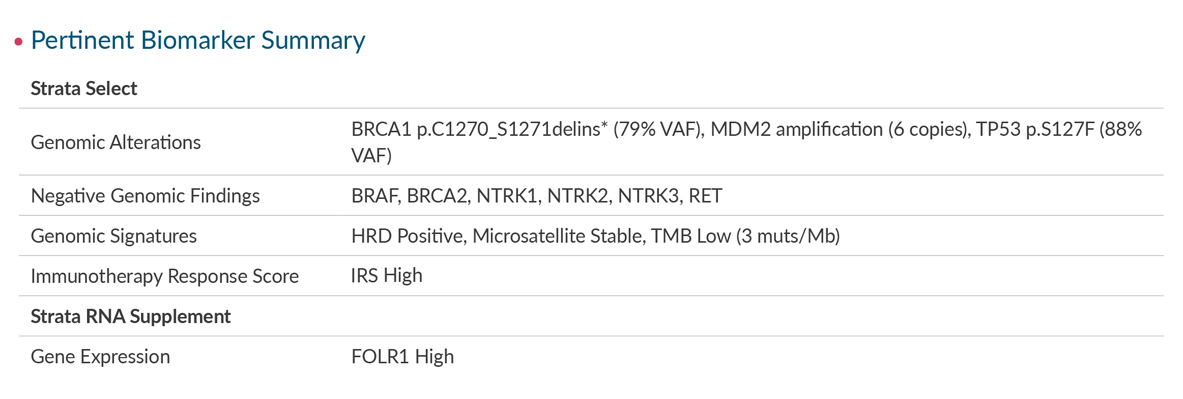
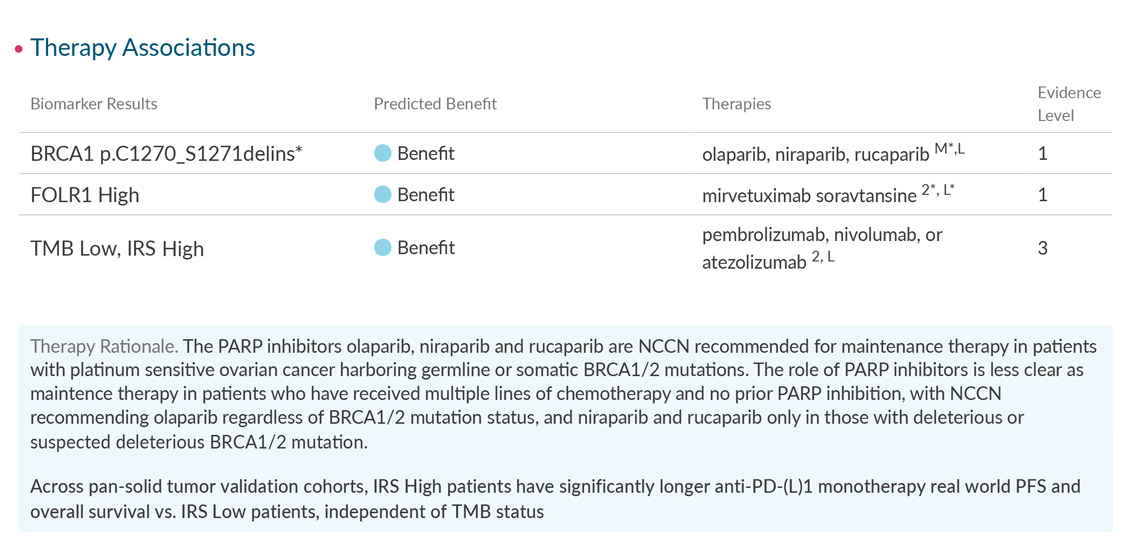
The Strata Select report includes the Strata Oncology Testing Summary, a consolidated view of all patient results enhanced with the expert pathologist commentary you need to put the results in context.
We know every patient is an individual, and our reports empower you to give each one of them their best possible therapy.
Input flexibility
The Strata Oncology platform is proven to provide results from small and challenging tumor tissue specimens, including:
- Diagnostic and metastatic biopsies
- Fine needle aspirations
- Fluid cytology
Leading performance
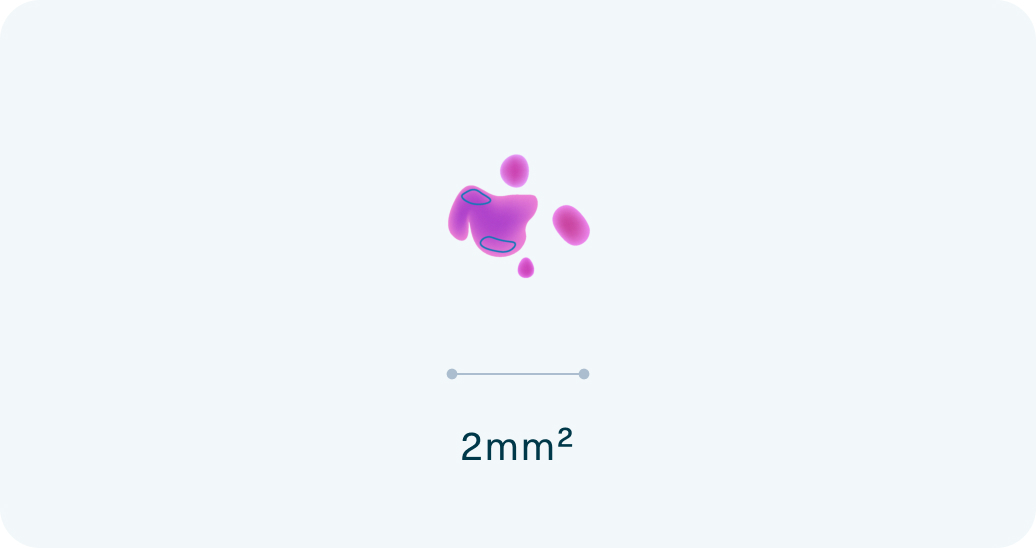
Ultra-low tumor tissue requirements
The Strata Select test requires 10 times less tissue than conventional comprehensive genomic profiling (CGP) tests – just 2mm2 tumor tissue.
Ultra-low tumor tissue requirements
The Strata Select test requires 10 times less tissue than conventional comprehensive genomic profiling (CGP) tests – just 2mm2 tumor tissue.
Proven performance on real-world tumor samples
In a study of more than 30,000 consecutive tissue samples from 28+ diverse US health systems, more than double the number of patients could be tested with Strata Select compared to conventional CGP tests1.
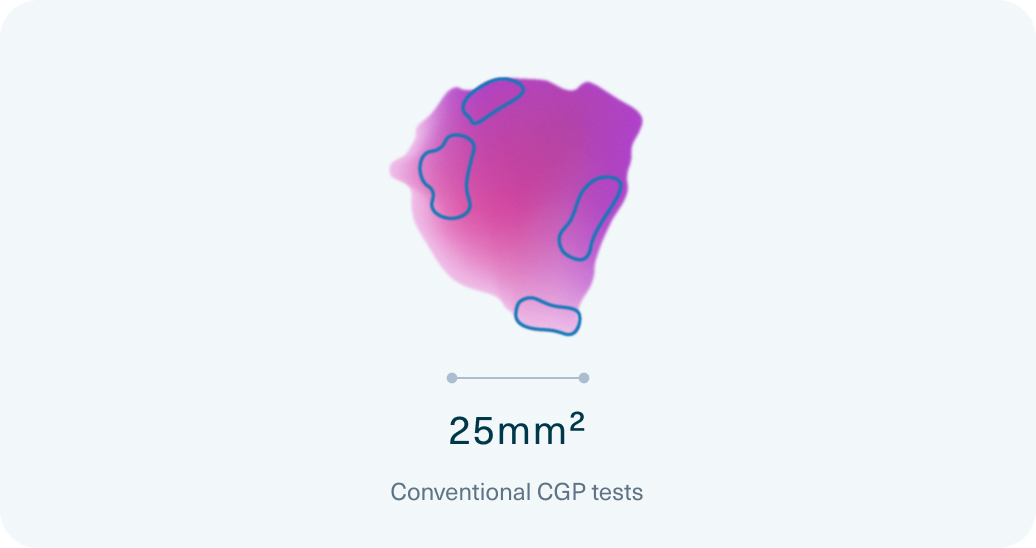
Conventional CGP
Only 41% of samples
met the minimum tumor surface area requirement (>25mm2) for conventional hybrid capture-based CGP tests
Strata Select
More than 94% of samples
were successfully reported by Strata’s PCR-based platform, regardless of sample size

Patient financial assistance
Strata Cares:
We’re here to help
At Strata Oncology, we believe every patient with cancer deserves to know if their tumor harbors actionable alterations.
Our Strata Cares™ Financial Assistance program strives to ensure affordable access to testing for patients and their families.
We offer generous financial assistance which may reduce or eliminate out-of-pocket cost for qualified patients in the U.S.
After you order testing for your patient, a Strata Patient Support Specialist will work directly with them to determine if they qualify for financial assistance.
Patients can apply for financial assistance before, during, and after the testing process. The final patient amount will be determined after all applicable insurance claims and appeals have been completed.
Applications for financial assistance can be made online.
Medicare Part B Coverage Criteria
- Patient has been diagnosed with a solid malignant neoplasm; AND
- Patient has either recurrent, relapsed, refractory, metastatic, or advanced stage III or IV cancer (only requires one of these to be met); AND
- Patient has not been previously tested with the same test using NGS for the same cancer genetic content; AND
- Patient has decided to seek further cancer treatment (e.g., therapeutic chemotherapy)